Is aluminum an acceptable material for a waterblock?
Moderators: NeilBlanchard, Ralf Hutter, sthayashi, Lawrence Lee
Is aluminum an acceptable material for a waterblock?
I'm going to WC my PC, and I will be making my own water block because I'm poor and like making things.
I'm having a hard time finding copper locally, but I have several nice pieces of aluminum. I know copper's thermal conductivity is much higher than Al, but the stock HSF (AMD 3700) is aluminum after all...
I'm having a hard time finding copper locally, but I have several nice pieces of aluminum. I know copper's thermal conductivity is much higher than Al, but the stock HSF (AMD 3700) is aluminum after all...
-
Qwertyiopisme
- Posts: 237
- Joined: Tue Nov 11, 2003 6:48 am
- Location: Gothenburg, Sweden
- Contact:
Aluminum will work fine, the thermal conductivity, though lower, is probably still good enough. (just as long as you don't have a prescott or anything)
And even if you could get ahold of copper, machining it is a real PITA (I speak from experience).
I would recomend going to forums.procooling.com and looking in the waterblock design/construction subforum, where there is a lot of good information (waterblock designs, mounting hole size/distances and so on).
And even if you could get ahold of copper, machining it is a real PITA (I speak from experience).
I would recomend going to forums.procooling.com and looking in the waterblock design/construction subforum, where there is a lot of good information (waterblock designs, mounting hole size/distances and so on).
Not exactly. To get real battery effect you need to have electrical current in both ends and some potential difference. Yes, mixing metals will cause them to oxidize faster, but it's nothing dramatic, especially when using de-ionized water.Aris wrote:just make sure your radiator isnt copper, or you risk turning your entire cooling system into a battery that will literally disolve your rad and waterblocks
And considering how many water-cooling parts are made if anodized aluminium, aluminium oxide might not be that poor thermal conductor anyway.
The physicists I know have told me that with de-ionized water, the computer parts in your rig will be obsolete before the aluminium parts are oxidized too badly to be of use..
And yes, I feel your pain with copper supply: It's terribly hard to get small amounts of copper. If you tell us where you live, we might be able to give some hints, thou.
OT, but Zds if you have trouble finding copper in Finland visit http://www.terasrenki.com/ and give the old geezer a call. He will find copper for you in pretty much any shape or size.zds wrote: And yes, I feel your pain with copper supply: It's terribly hard to get small amounts of copper. If you tell us where you live, we might be able to give some hints, thou.
Finding what you need could take a while. I had to wait about two weeks for the guy to come up with a 90mm diameter and 30cm long copper rod.
Thanks a lot for a hint!Vihta wrote:OT, but Zds if you have trouble finding copper in Finland visit http://www.terasrenki.com/ and give the old geezer a call. He will find copper for you in pretty much any shape or size.
Some points:zds wrote:Not exactly. To get real battery effect you need to have electrical current in both ends and some potential difference. Yes, mixing metals will cause them to oxidize faster, but it's nothing dramatic, especially when using de-ionized water.Aris wrote:just make sure your radiator isnt copper, or you risk turning your entire cooling system into a battery that will literally disolve your rad and waterblocks
And considering how many water-cooling parts are made if anodized aluminium, aluminium oxide might not be that poor thermal conductor anyway.
The physicists I know have told me that with de-ionized water, the computer parts in your rig will be obsolete before the aluminium parts are oxidized too badly to be of use..
And yes, I feel your pain with copper supply: It's terribly hard to get small amounts of copper. If you tell us where you live, we might be able to give some hints, thou.
1. The water will ionize almost immediately in a typical watercooling loop. Deionized water will strip ions from any metallic surface it comes in contact with very quickly.
2. You WILL get galvanic corrosion. It will not take years to appear either. Anodizing will slow the process down significantly, as will running anti-corrosive additives (anti-freeze or the like). Here's what can happen after just 6 months of unprotected running:
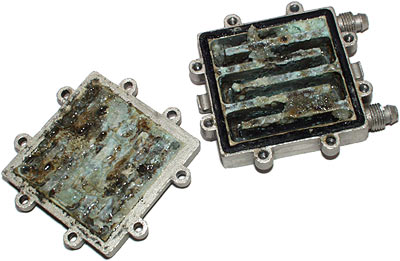
You don't want that in your waterloop.
NB: The electrical potential that causes the "battery effect" is from the metals themselves. You don't need anything except copper, aluminium and a suitable electrolyte (water does just fine) for corrosion to occur.
If you're having trouble sourcing copper, you might want to try brass. It's not as good as Al or Cu, but it won't corrode and it's easy to machine. Alternatively, make sure you get an Al radiator and make Al blocks.
-
jamesavery22
- Posts: 271
- Joined: Thu Jun 24, 2004 3:19 pm
Butcher beat me to it =)
Just have a few additions.
Even if you have all Al in your loop it will still corode... Aluminum still oxidizes by itself. It just doesnt oxidize like other metals do. It oxidizes something that actually coats itself and prevents further oxidization. I read that somewhere that was talking about aluminum siding.
A pure Al still needs anticorrosive agents. Zerex racing coolant is designed for Al block engines and all copper rads. Just use that. Or 80/20 Distilled/AF.
Just have a few additions.
Even if you have all Al in your loop it will still corode... Aluminum still oxidizes by itself. It just doesnt oxidize like other metals do. It oxidizes something that actually coats itself and prevents further oxidization. I read that somewhere that was talking about aluminum siding.
A pure Al still needs anticorrosive agents. Zerex racing coolant is designed for Al block engines and all copper rads. Just use that. Or 80/20 Distilled/AF.
Do you know the thermal conductivity of the aluminum you have? Trace amounts of other elements can dramatically reduce the thermal conductivity of both aluminum and copper. Hence, even aluminum and copper that's sold as "pure" can have relatively low thermal conductivity. Fortuantely, there are standards for this, and if you know what you've got, it shouldn't be too hard to look it up on the web. Here's one site I've found useful.
http://www.matweb.com/search/SearchProperty.asp?e=1
If you don't know what you have, I wouldn't put too much time into milling waterblocks until you've done some tests. If you've got an alloy with low thermal conductivity, all your blocks will be duds no matter how good the design and craftsmanship.
http://www.matweb.com/search/SearchProperty.asp?e=1
If you don't know what you have, I wouldn't put too much time into milling waterblocks until you've done some tests. If you've got an alloy with low thermal conductivity, all your blocks will be duds no matter how good the design and craftsmanship.
